Characterization and comparison of receptor orthologues using bioSensAll®
Animal studies are a key component of the preclinical stage of drug development. Such studies involve animal (often rodent) models of human disease in which a drug’s safety and efficacy are evaluated prior to testing in humans. Based on the phylogenetic relatedness and physiological similarities between rodents and humans, it has long been assumed that a drug’s safety and activity profile in rodents is predictive of that in humans. Yet, translation of preclinical rodent model data to successful human clinical trial outcomes is modest, with only ~10% of drugs entering Phase I clinical trials ultimately being approved for clinical use [1]. Factors accounting for this low translation rate include poor study methodology and failure of animal models to accurately mimic human disease, with the latter issue rooted in all-toooften neglected genetic, molecular, immunologic and cellular differences between animals and humans.
In this application note, we demonstrate how bioSensAll® can be used early in the drug development process to screen for inter-orthologue differences in target GPCR signaling and pharmacology.
Results and Conclusion
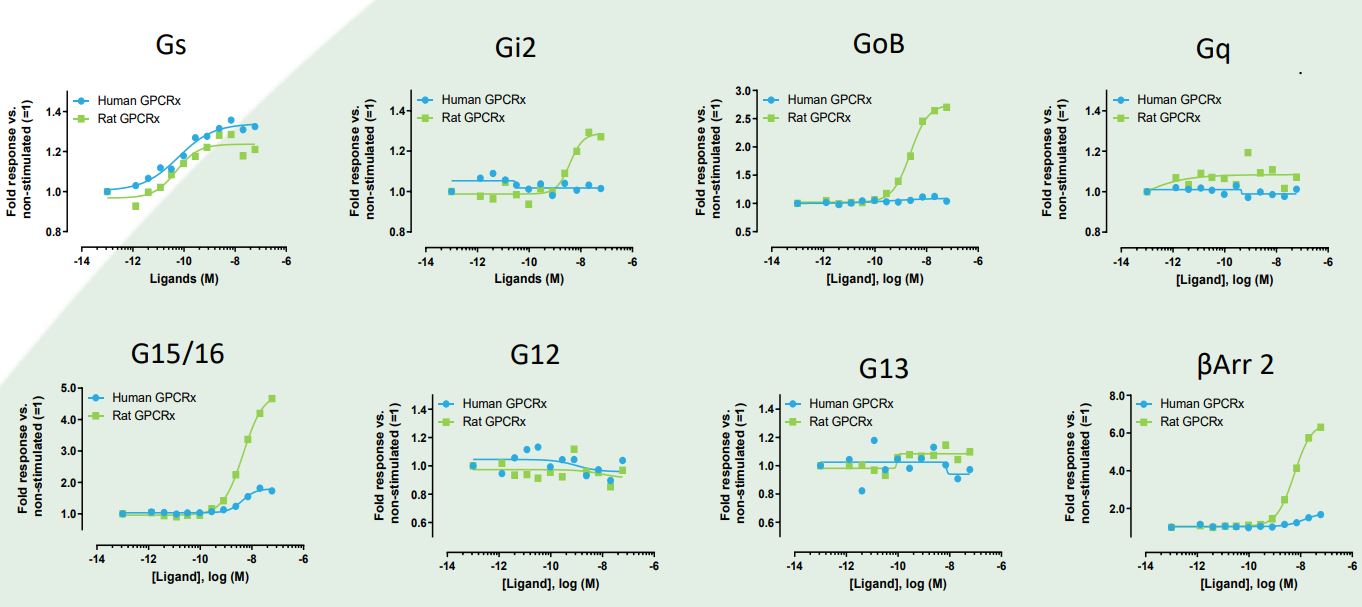
The signaling profiles of rat and human orthologues of an undisclosed GPCR (GPCRx) were characterized using 8 pathway-specific bioSensAll® sensors. The data revealed that in response to their endogenous ligand, rat and human GPCRx were equally active in engaging Gɑs.However, significant inter-species differences were observed
for the activation of Gɑi2, GɑoB, Gɑ15/16 and ß-arrestin 2, with Gi-family G proteins Gɑi2 and GɑoB being engaged uniquely by the rat receptor. Such information, when generated early in the drug development process, would allow for all necessary and appropriate modifications to the study design so as to reduce attrition risks. This information would be particularly invaluable to all drug development campaigns in
which biased ligands are being sought and where animal models are used to expose and/or study the therapeutic implications of biased signaling. As demonstrated in the example herein, GPCR signaling profiles, and thus biased signaling, can be speciesspecific.
bioSensAll® is a versatile technology that can be used to differentiate the signaling and pharmacology of orthologous GPCRs. This information, which can be rapidly and easily derived with the bioSensAll® platform, may have an important impact on the success of drug development programs.
References
[1] Hay, M., Thomas, D.W., Craighead, J.L., Economides, C., Rosenthal, J. (2014) Clinical development success rates for investigational drugs. Nat Biotechnol. 32(1):40-51