Discovery and characterization of functional antibodies using bioSensAll®
Receptor-targeting molecules a Antibodies represent a fastgrowing class of therapeutic agents offering numerous advantages over more conventional small molecule approaches. Notably, antibodies provide superior selectivity and specificity, better pharmacokinetics, and a capacity to induce depletion of specific cell populations. Because of these inherent advantages and their superior success rate in all drug
development phases, there is a strong interest in developing antibodies capable of targeting specific surface receptors involved in pathological processes. G protein-coupled receptors (GPCRs) play central roles in various pathologies and are targeted by ~30% of small molecule pharmaceuticals. Accordingly, there is a growing interest for developing therapeutic antibodies targeting GPCRs. However, the
development of therapeutic anti-GPCR antibodies has been plagued by the conformational instability of GPCRs (i.e.,complicating the preparation of native and functional forms of antigens for immunization) as well as the lack of a suitable platforms for the selection of functional antibodies. Today, most antibody screening and selection strategies focus on antigen binding affinity, which does not necessarily translate
into biological activity (i.e., agonist, antagonist or allosteric action). bioSensAll® consists of a set of 25 BRET-based, signaling pathway-specific biosensors that permit a high throughput assessment of GPCR
antibody function. This technology allows for antibody mode of action studies as well as the characterization of antibody signaling signatures. Importantly, these signaling signatures can be linked to clinical efficacy and, potentially, safety, as the activation of certain pathways is either correlated to beneficial or undesirable effects.
Results and Conclusion
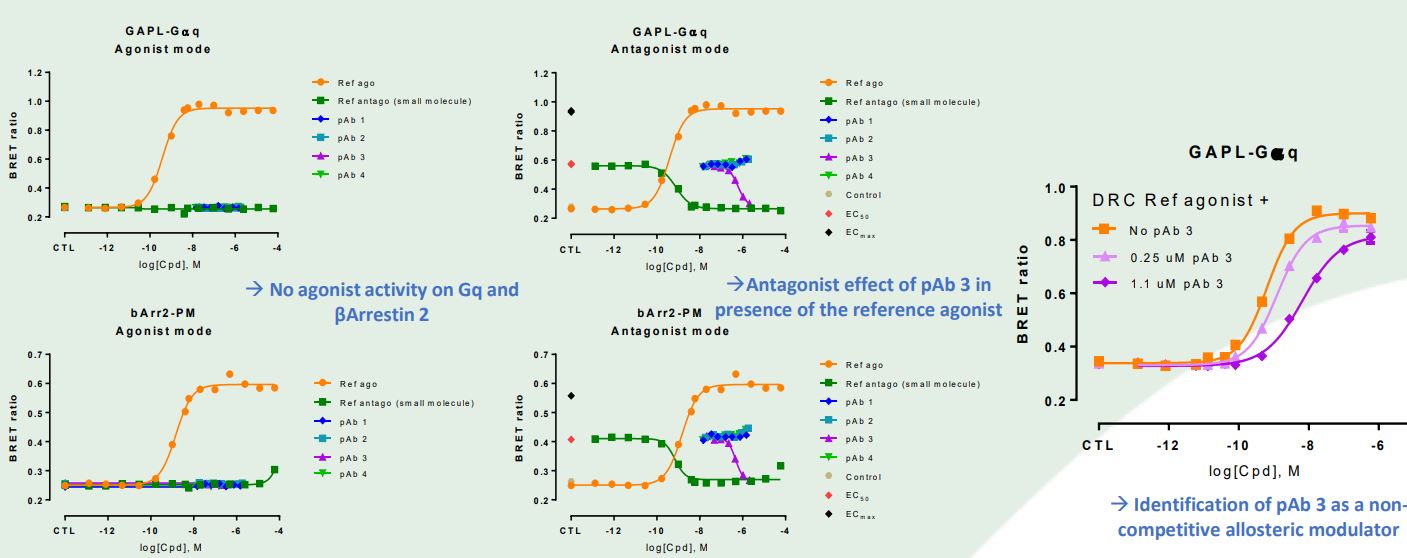
Two purified polyclonal antibodies (pAb) developed by Immune Biosolutions were tested in agonist and
antagonist/allosteric modes using the Gq and βArrestin-2 biosensors. pAb 3 reversed the ability of an Ec50 of reference agonist to engage both pathways and was as efficacious as the reference small molecule antagonist. Schild plot analysis of pAb 3 revealed an antibody dose-dependent decrease in potency and efficacy of reference agonist-induced Gq activation. The latter result is typical ofa negative
non-competitive allosteric modulator. bioSensAll® allows for the identification of functional antibodies via interrogation of their effects on a broad spectrum of target-associated signaling pathways.