Measurement of pathway-specific signaling kinetics in real-time with bioSensAll®
Comprehension of the temporal aspects (i.e., kinetics and dynamics) of receptor-mediated signaling is integral to fully appreciating the mechanisms by which extracellular signals impact cellular behavior. For ligand-receptor interactions, kinetics can be generally described at two levels: i) ligand binding to the target receptor, and ii) receptor downstream signaling. Ligand binding kinetics are defined in terms of kon and koff values (ligand association and dissociation rates, respectively) determined via radioligand
binding assays. Signaling kinetics are defined in temporal terms and may range from fractions of a second to many hours. The effect of kinetics on a ligand’s efficacy and biological action(s) has long been recognized but all too often overlooked [1]. Consideration of kinetics is all the more important when dealing with G-protein coupled receptors (GPCRs) given their well appreciated capacity to activate multiple signaling pathways exhibiting distinct kinetic properties. Further, it is now evident that
different ligands of a given GPCR can activate distinct subsets of receptor-coupled signaling cascades, thus leading to compoundspecific signaling signatures (a.k.a., biased agonism). Recent observations have suggested that both a ligand’s signaling signature and biased qualities may be qualitatively and quantitatively modulated over time [2]. Single point readouts of signaling conducted at equilibrium may therefore provide an incomplete portrait of a ligand’s character and fail to capture important
differentiating elements between compounds. As a result, the ability to measure signaling kinetics in real-time over short (milliseconds) and extended (hours) timescales offers invaluable insight into a compound’s pharmacological properties. In this application note, we demonstrate how a single, multiplecompound addition assay with bioSensAll® biosensors was used to obtain real-time kinetic measurements of G-protein (GoA) activation and ß-arrestin 2 membrane recruitment for the human
mu-type opioid receptor (hMOR) agonist and antagonist DAMGO and naloxone, respectively.
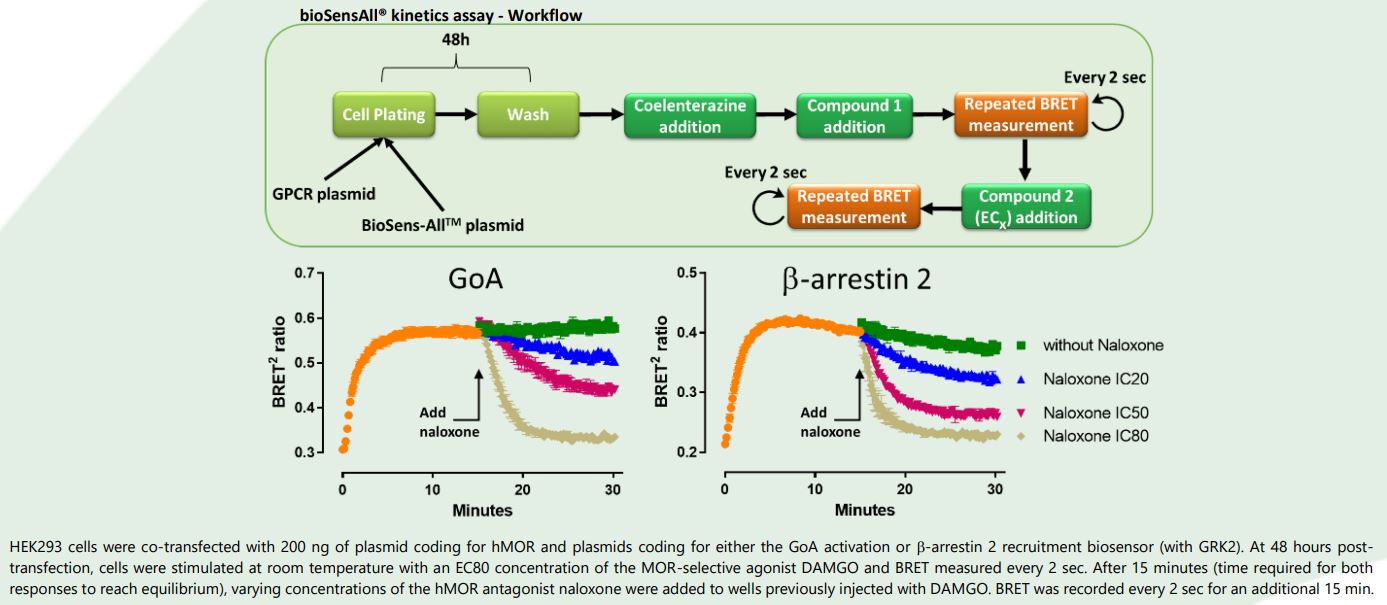
Results and Conclusion
DAMGO-induced GoA activation and plasma membrane recruitment of β-arrestin 2 displayed comparable kinetics post-agonist addition. However, whereas maximal GoA activation was sustained for at least 30 min, the presence of β-arrestin 2 at the plasma membrane declined in a time-dependent manner. Pathway-specific differences in sensitivity to the antagonist activity of naloxone were also revealed. Indeed, naloxone displayed faster kinetics in antagonizing DAMGO-dependent β-arrestin 2 membrane localization (especially visible with naloxone IC50). bioSensAll® technology allows for the realtime assessment of ligand signaling kinetics and dynamics across multiple GPCR-downstream signaling pathways and on timescales ranging from milliseconds to hours in a simple and homogenous
assay format.
bioSensAll® thus provides additional information about ligand pharmacology that cannot be obtained via equilibrium or endpoint assays.
References
[1] Mackay, D. (1977) A critical survey of receptor theories of drug action. Kinet. Drug Action. 47:225–321. [2] Klein Herenbrink, C., Sykes, D.A., Donthamsetti, P. et al. (2016) The role of kinetic context in apparent
biased agonism at GPCRs. Nat. Commun. 7. Article number: 10844.