BRET biosensors allowing the spatio-temporal moniroting of β-arrestins
How the biosensors work
β-arrestins are multifunctional adaptor proteins classically associated with the “arrest” of G protein-coupled receptor (GPCR)-mediated signaling by promoting receptor desensitization and internalization. However, it is now evident that β-arrestins (mainly β-arrestin 1 and β-arrestin 2) can also scaffold signaling complexes proximal to a given GPCR and modulate the activity of various signaling networks (including ERK1/2, NF-κB, and PI3-K). Importantly, β-arrestin-mediated signaling may be spatially and temporally distinct, and result in different biological outcomes, compared to G protein-mediated signal transduction. Consequently, GPCR-downstream signaling can occur via G protein-dependent and/or β-arrestin-dependent (G protein-independent) mechanisms (1-2). β-arrestins are recruited to activated GPCRs following receptor phosphorylation by G protein-coupled receptor kinases (GRKs) and/or other protein kinases (e.g., PKA, PKC). GPCRs are broadly categorized into two classes based on the stability of their interaction with β-arrestin proteins. Class A GPCRs (e.g., mu opioid receptor, dopamine D1A receptor) generally form transient complexes with β-arrestins at the plasma membrane, internalize into endosomes without β-arrestins and are rapidly recycled to the plasma membrane. Conversely, Class B receptors (e.g., angiotensin II type 1A receptor, vasopressin V2 receptor) form longer-lived complexes with β-arrestins, stably internalize into endosomes with their associated β-arrestins and display slower recycling kinetics (3).
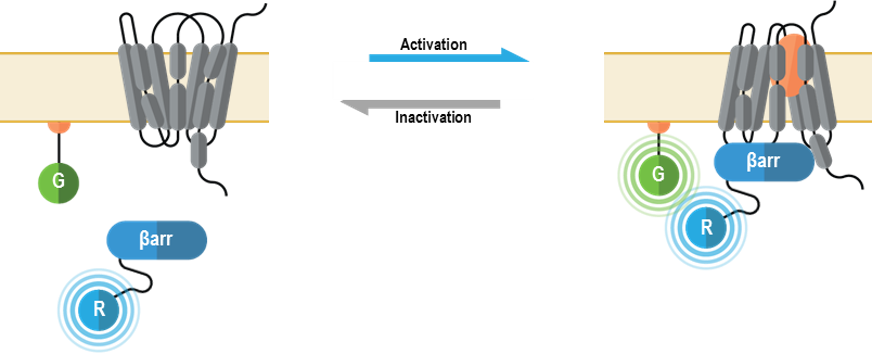
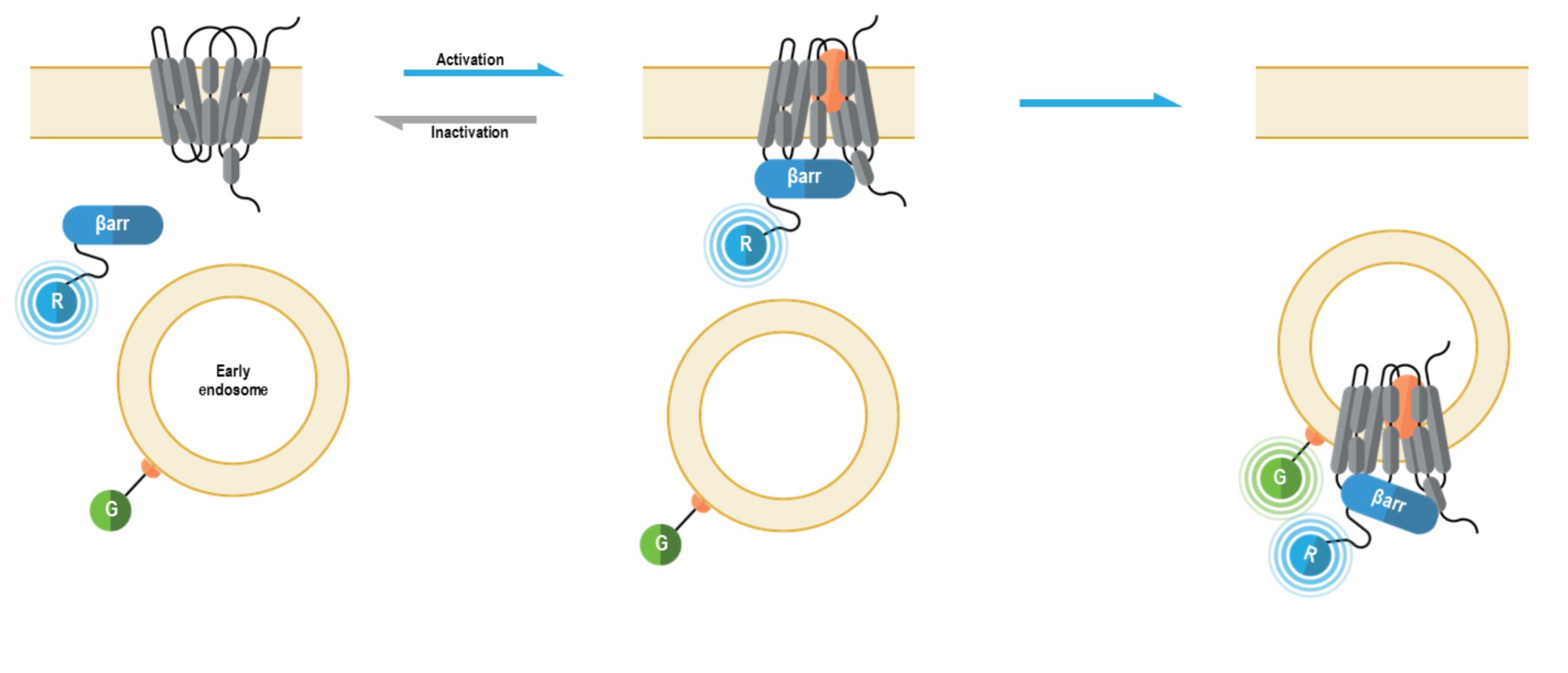
The bioSens-All® β-arrestin membrane recruitment sensors allow for real-time spatio-temporal monitoring of β-arrestins following GPCR activation. The multimolecular β-arrestin biosensors consists of a β-arrestin 1 or 2 fused at their C-terminus to Renilla luciferase (RLuc; R in figure below) that translocates to the plasma membrane anchored green fluorescent protein (GFP; G in following figure) or early endosome anchored GFP. Specifically, these sensors were designed to detect the recruitment of β-arrestin proteins to either the plasma membrane or early endosomes, with localization to each compartment resulting in an increased BRET signal (4).
Beta Arrestin Plasma Membrane recruitment biosensor:
Beta Arrestin Early Endosome recruitment biosensor:
β-arrestin recruitment data generated with the Glucagon-like peptide-1 receptor
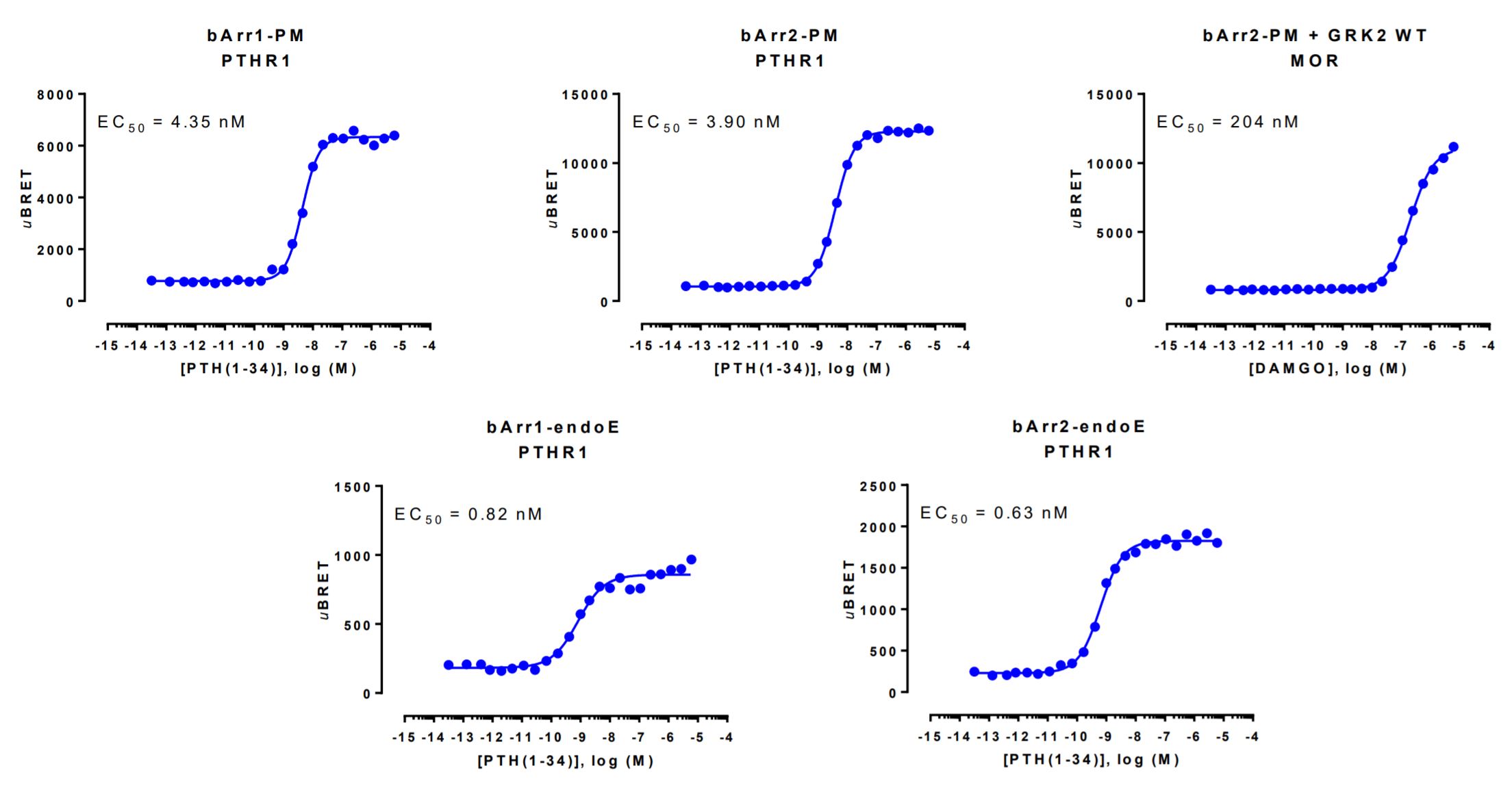
HEK293 cells were transfected with a receptor coding plasmid (human parathyroid hormone type 1 receptor (PTHR1) or the human mu opioid receptor (MOR)) as well as plasmids coding for the β-arrestin membrane recruitment biosensor or early endosome recruitment biosensor.
The MOR-mediated plasma membrane recruitment of β-arrestin 2 was assessed in the presence of overexpressed WT GRK2. On the day of BRET, cells were rinsed with assay buffer, incubated with coelenterazine and increasing amounts of PTH(1-34) or DAMGO for 10 minutes (β-arrestin-PM sensors) or 30 minutes (β-arrestin-endoE sensors) and BRET subsequently measured.