The power of BRET to study conformational changes within β-arrestins
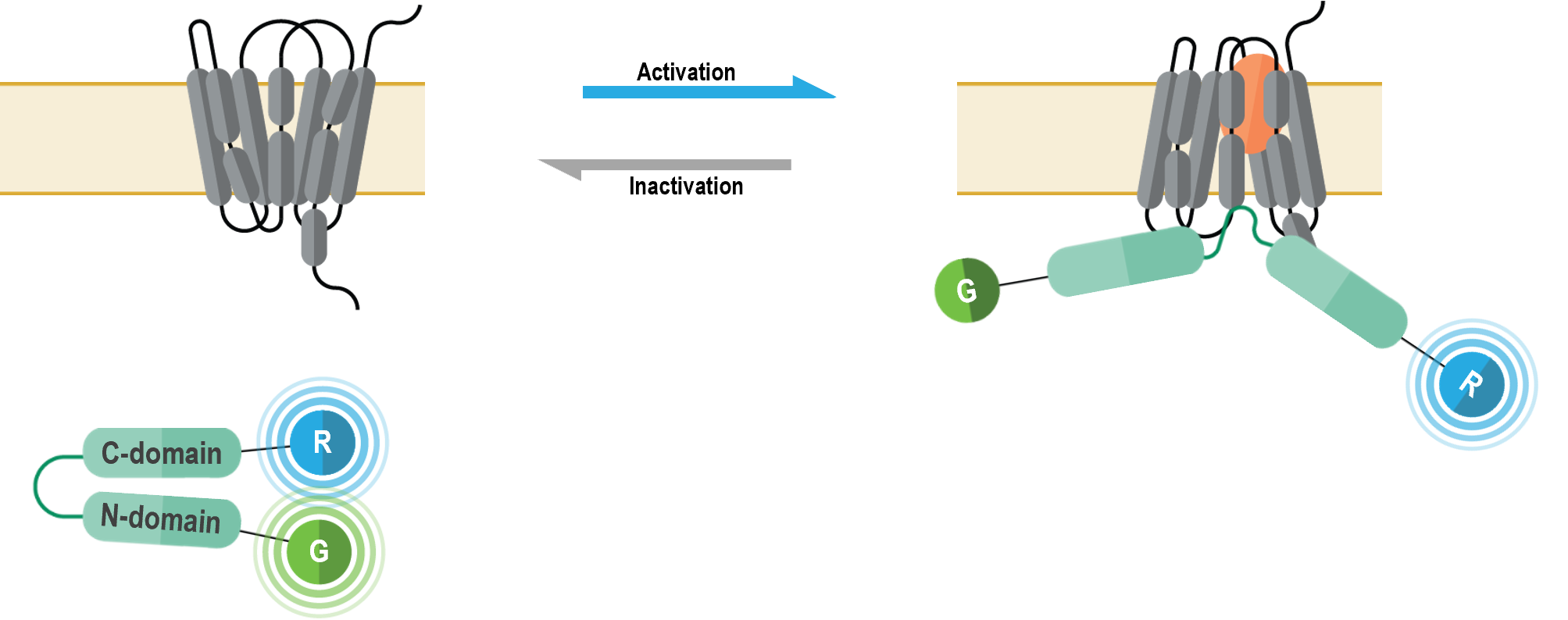
How the biosensor works
β-arrestins are multifunctional adaptor proteins classically associated with the “arrest” of G protein-coupled receptor (GPCR)-mediated signaling by promoting receptor desensitization and internalization. However, it is now evident that β-arrestins (mainly β-arrestin 1 and β-arrestin 2) can also scaffold signaling complexes proximal to a given GPCR and modulate the activity of various signaling networks (including ERK1/2, NF-κB, and PI3-K). Importantly, β-arrestin-mediated signaling may be spatially and temporally distinct, and result in different biological outcomes, compared to G protein-mediated signal transduction. Consequently, GPCR-downstream signaling can occur via G protein-dependent and/or β-arrestin-dependent (G protein-independent) mechanisms (1-2). β-arrestins are recruited to activated GPCRs following receptor phosphorylation by G protein-coupled receptor kinases (GRKs) and/or other protein kinases (e.g., PKA, PKC). Different ligands acting on a given receptor are believed to produce unique receptor phosphorylation patterns (i.e., phosphorylation “barcodes”), resulting in the stabilization of different receptor-associated (active) β-arrestin conformations. Furthermore, each conformation is suggested to exhibit distinctive functional properties, thus adding an extra qualitative dimension to GPCR-downstream β-arrestin-dependent signaling (2-3).
The bioSensAll® β-arrestin double brilliance conformation biosensors are unimolecular BRET-based biosensors that monitor conformational changes occurring within β-arrestins upon GPCR activation. These biosensors consist of β-arrestin 1 or β-arrestin 2 proteins with an N-terminal green fluorescence protein (GFP; G in following figure) tag and a C-terminal Renilla luciferase (RLuc; R in figure below) tag. Recruitment of β-arrestins to an activated receptor leads to a structural reorganization and subsequent physical separation of N-terminal and C-terminal regions of the β-arrestin proteins, ultimately resulting in a BRET signal decrease (4).
β-arrestin double brilliance conformation biosensor data
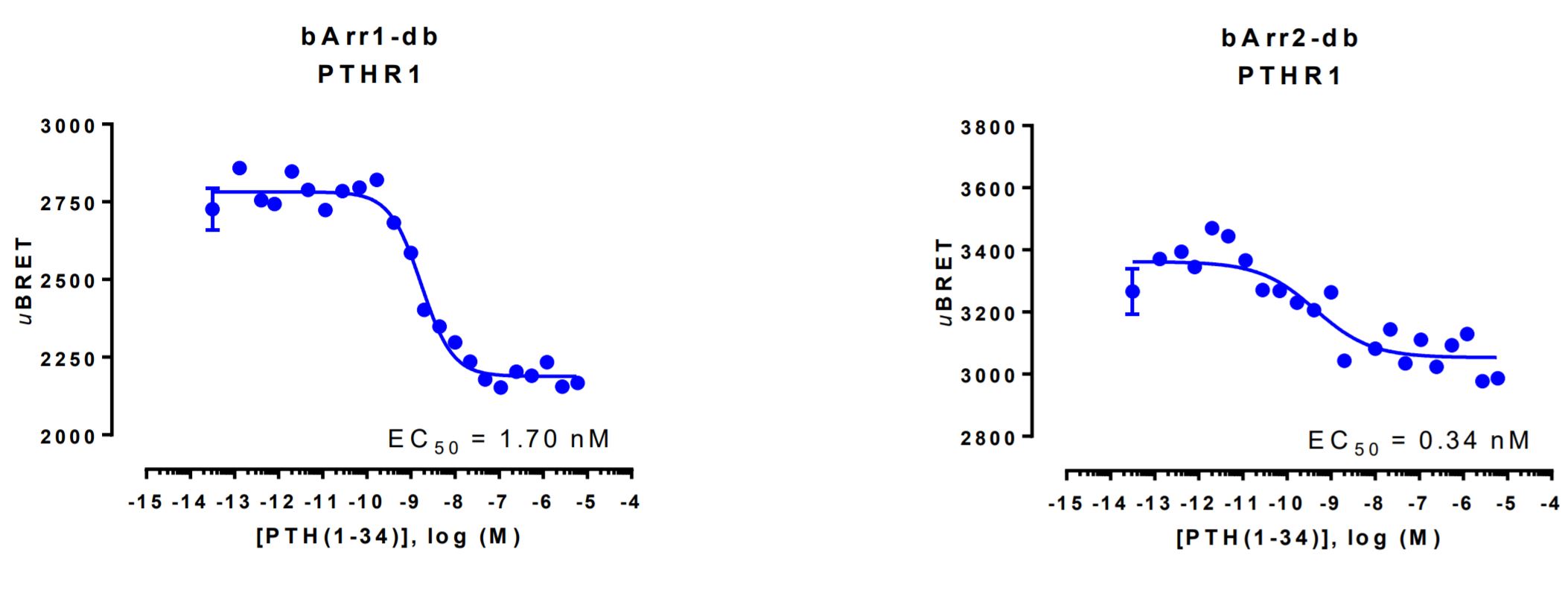
HEK293 cells were transfected with a receptor coding plasmid (human parathyroid hormone type 1 receptor (PTHR1)) in addition to the plasmid coding for the β-arrestin double brilliance conformation biosensor.
On the day of BRET, cells were rinsed with assay buffer, incubated with coelenterazine and increasing amounts of PTH(1-34) for 10 minutes and BRET subsequently measured.