Secondary messenger monitoring: The PIP3 biosensor
How the biosensor works
Phosphatidylinositol-3,4,5-triphosphate (PIP3) is a plasma membrane phospholipid that serves as a second messenger in signal transduction. Generation of PIP3 occurs via class I phosphoinositol 3-kinase (PI3K)-induced phosphorylation of phosphatidylinositol-4,5-bisphosphate (PIP2). PI3K is activated following ligand binding to various receptors involved in numerous biological processes, including receptor tyrosine kinases, integrin receptors, cytokine receptors and some G protein-coupled receptors (GPCRs) (1). GPCR-downstream activation of PI3K is mediated by Gβ/γ subunits of heterotrimeric G proteins (2-3). Generation of PIP3 promotes the plasma membrane recruitment of proteins harboring the PIP3-binding pleckstrin homology (PH) domain. Pleckstrin homology domains are present in various proteins (e.g., Akt/PKB, PDK1 and GPCR kinases (GRKs) 2 and 3). PIP3-mediated signaling is terminated by phosphatases (e.g., PTEN or SHIPs) involved in the dephosphorylation of the second messenger (1).
The bioSens-All® multimolecular PIP3 sensor consists of a PH domain of AKT (PIP3-BD in the following figure) fused at the N-terminus of Renilla luciferase (RLuc; R in figure below) that translocates to the plasma membrane upon PIP3 production. Membrane recruitment of this biosensor translates into an increased BRET efficiency with a plasma membrane anchored green fluorescent protein (GFP; G in following figure). The magnitude of the resulting BRET signal is directly proportional to the level of PIP3 produced following receptor stimulation.
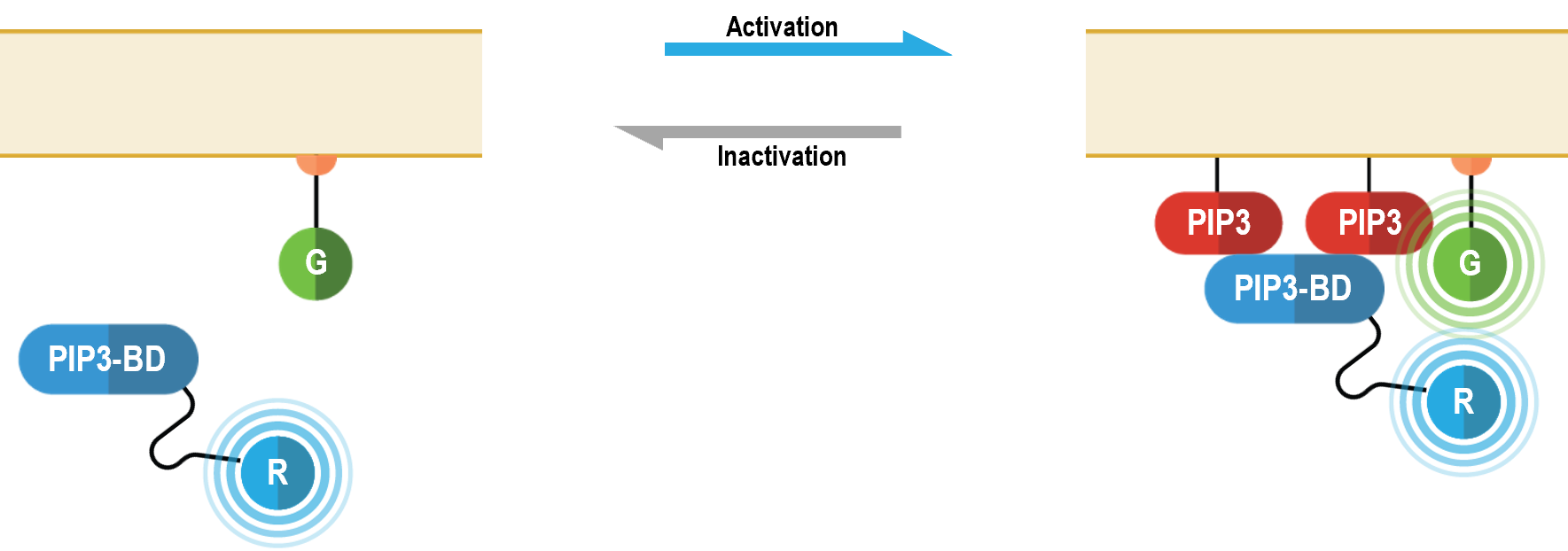
PIP3 data generated with EGFR
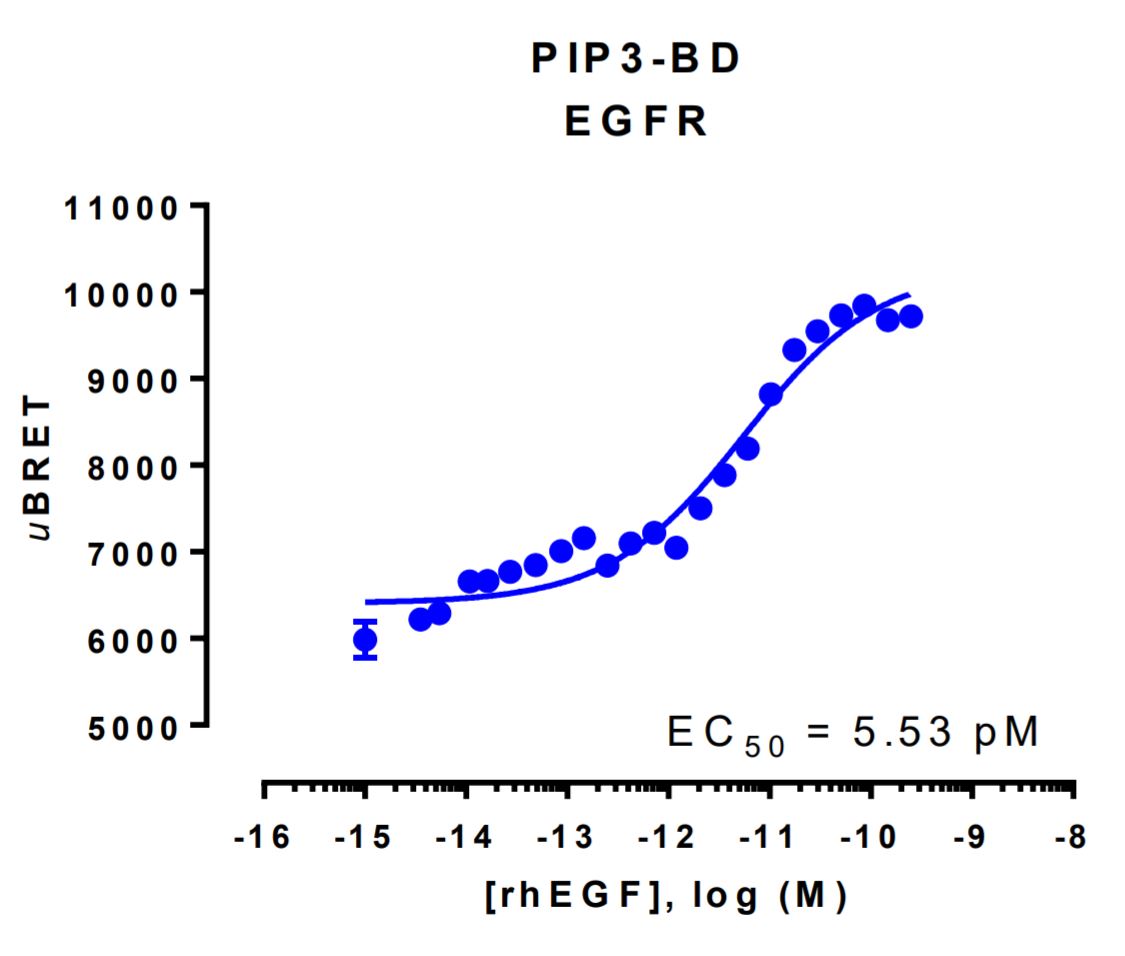
HEK293 cells were transfected with a receptor coding plasmid (human epidermal growth factor receptor (EGFR)) and plasmids coding for the PIP3 biosensor. On the day of BRET, cells were rinsed with assay buffer, incubated with coelenterazine and increasing amounts of rhEGF for 10 minutes and BRET subsequently measured.
References
1- Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. 2014. J Cell Sci. Mar 1;127(Pt 5):923-8. PMID: 24587488.
2- Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. 1997. J Biol Chem. Sep 26;272(39):24252-6. PMID: 9305878.
3- Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, Barrett MO, Waldo GL, Surve C, Hsueh C, Perisic O, Harteneck C, Shepherd PR, Harden TK, Smrcka AV, Taussig R, Bresnick AR, Nürnberg B, Williams RL, Backer JM. G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness. 2012. Sci Signal. Dec 4;5(253):ra89. PMID: 2003264.