How receptor trafficking and localization biosensors bring valuable information
How the biosensor works
Receptor trafficking and localization are critical determinants of a receptor’s biological activity and the pharmacological action(s) of ligands. Trafficking of G protein-coupled receptors (GPCRs) begins with their activation by ligands and their subsequent phosphorylation by various kinases, including PKA, PKC and (most prominently) GPCR kinases (GRKs). Phosphorylation usually occurs on serine and/or threonine residues located within the GPCR third intracellular loop and the carboxyl-terminal tail domain. Phosphorylated GPCRs then recruit multifunctional adaptor proteins β-arrestin 1 and β-arrestin 2. In addition to desensitizing GPCRs (i.e., promote uncoupling of receptors from associated heterotrimeric G protein(s)), β-arrestins facilitate GPCR endocytocis by recruiting to the receptor various elements of the endocytic machinery (e.g., clathrin, AP-2) (1). GPCRs are broadly categorized into two classes based on the stability of their interaction with β-arrestin proteins. Class A GPCRs (e.g., mu opioid receptor, dopamine D1A receptor) generally form transient complexes with β-arrestins at the plasma membrane, internalize into endosomes without β-arrestins and are rapidly recycled to the plasma membrane. Conversely, Class B GPCRs (e.g., angiotensin II type 1A receptor, vasopressin V2 receptor) form longer-lived complexes with β-arrestins, stably internalize into endosomes with their associated β-arrestins and display slower recycling kinetics (2). In addition to the aforedescribed clathrin- and β-arrestin-dependent endocytocis, GPCRs have also been suggested to undergo β-arrestin-independent endocytocis that is either clathrin-dependent or independent (1, 3-4).
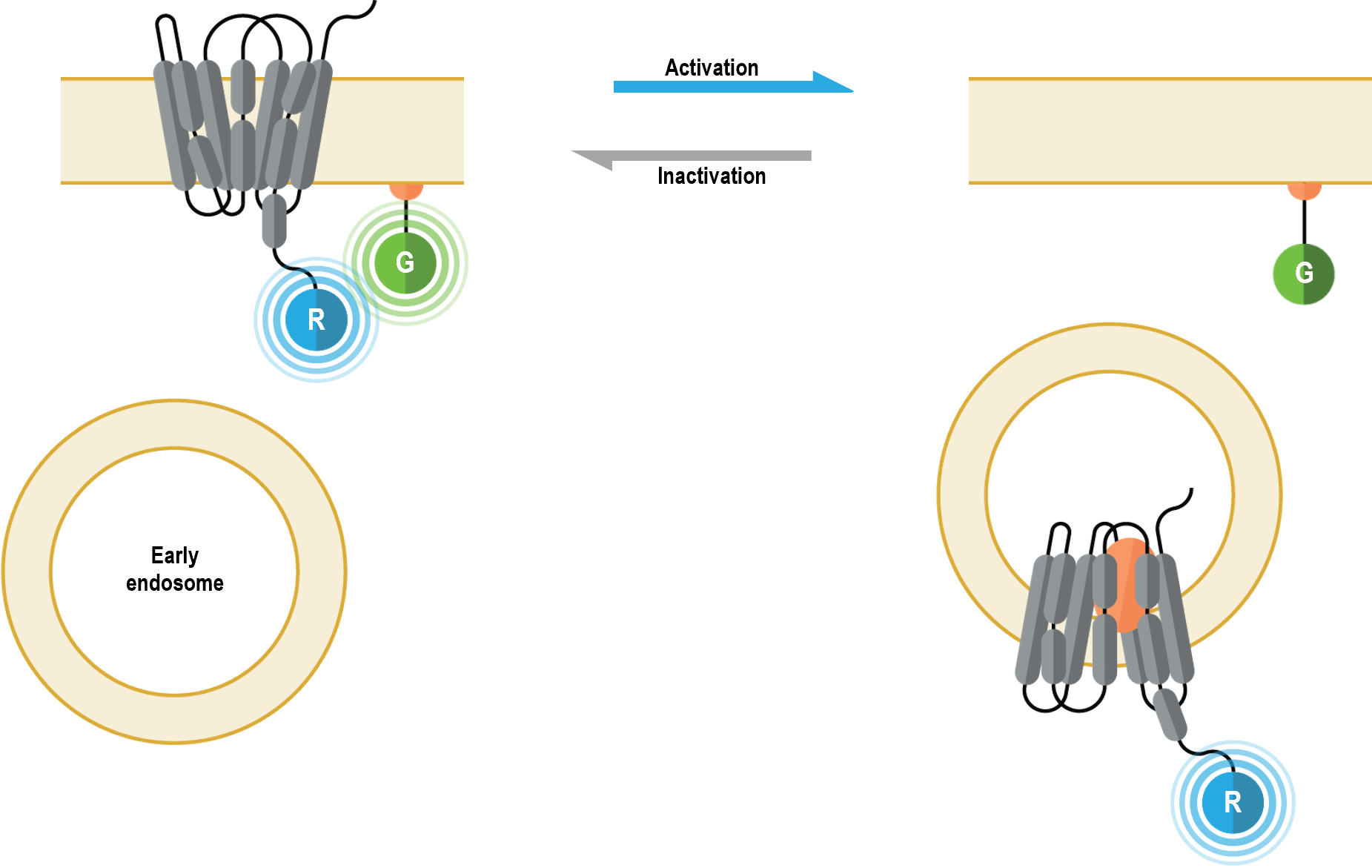
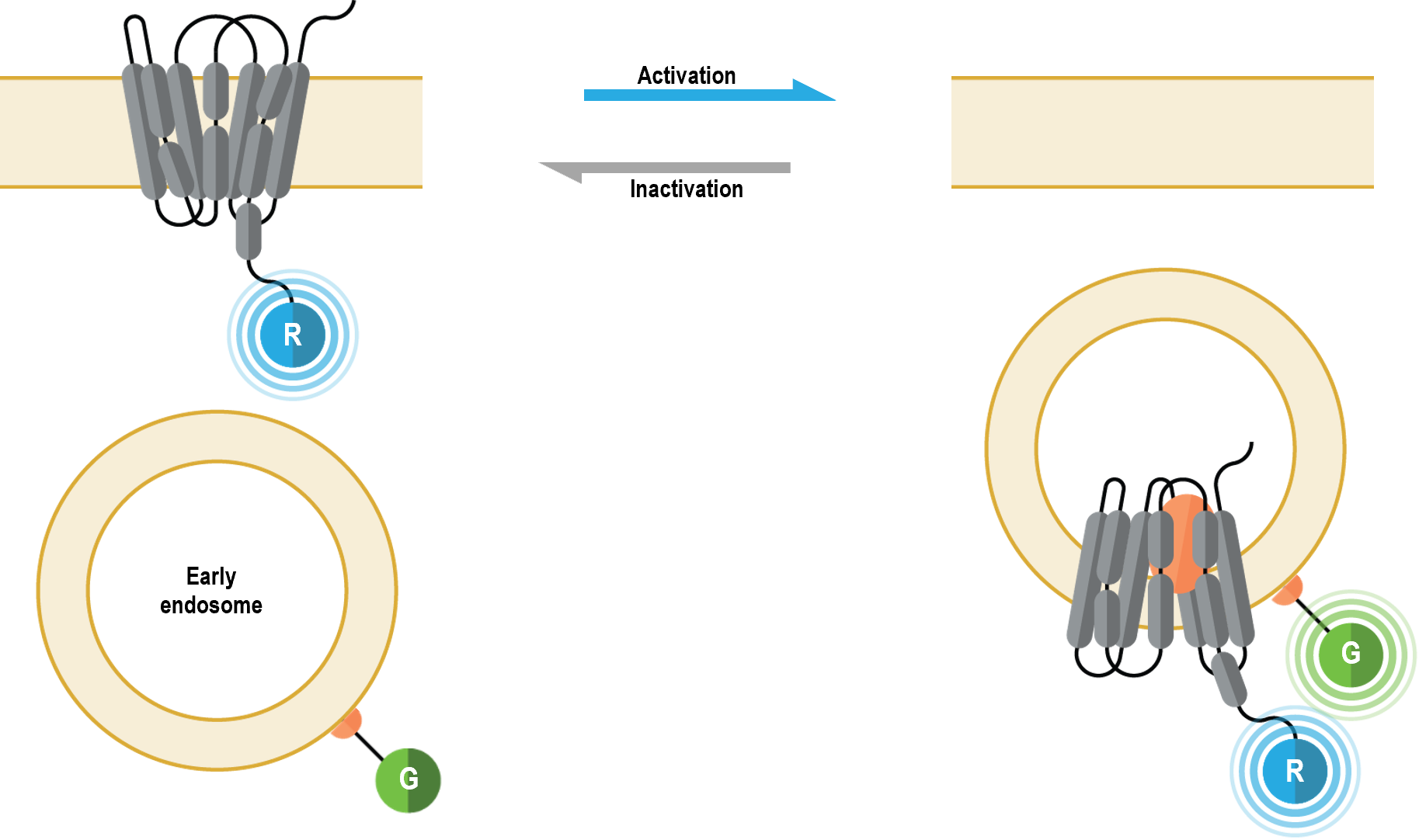
The bioSens-All® receptor trafficking sensors allow for real-time spatio-temporal monitoring of GPCRs following their activation. Specifically, these multimolecular sensors were designed to detect GPCR localization at the plasma membrane (PM) or in early endosomes (5). The receptor trafficking biosensors consist of a GPCR of choice fused at its C-terminus to Renilla luciferase (RLuc; R in figure below). Translocation of the tagged receptor away from the PM results in a time-dependent distancing from the PM anchored green fluorescent protein (GFP; G in following figure), thus resulting in a decreased BRET signal. Conversely (and concomitantly), arrival of the tagged GPCR to the early endosome (also harboring membrane-anchored GFP) results in a time-dependent increase in BRET.
GPCR plasma membrane biosensor:
GPCR early endosome biosensor:
Spatio-temporal data generated with the Glucagon-like peptide-1 receptor
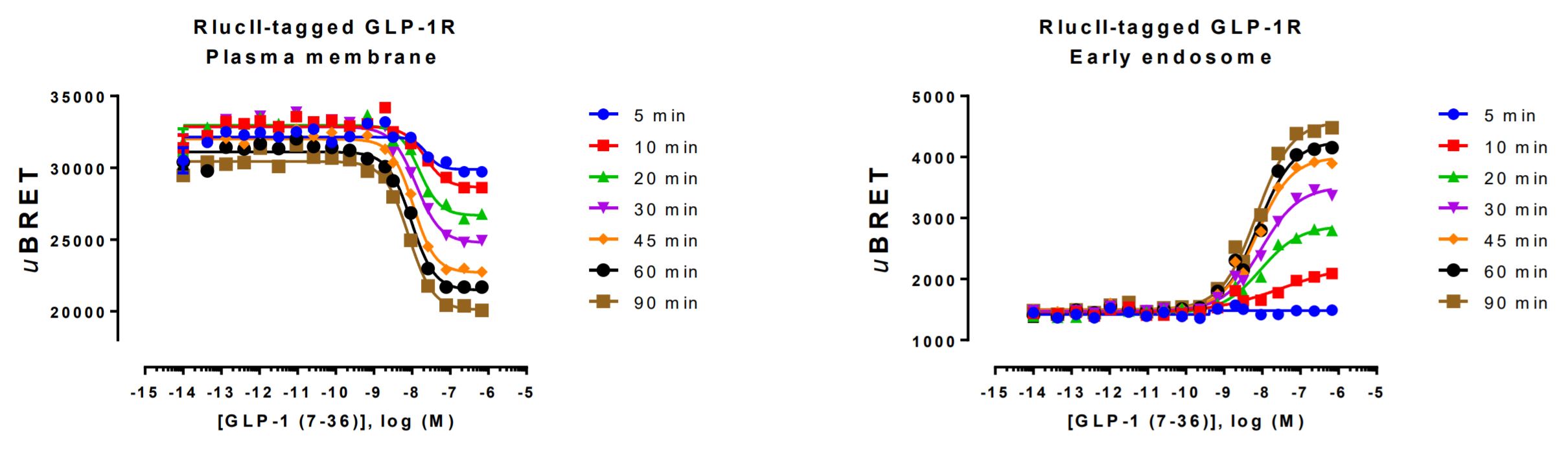
HEK293 cells were transfected with a plasmid coding for the human glucagon-like peptide 1 receptor (GLP-1R) tagged at its C-terminus with Renilla luciferase as well as the plasma membrane recruitment biosensor (left) or early endosome recruitment biosensor (right). On the day of BRET, cells were rinsed with assay buffer, incubated with coelenterazine and increasing amounts of GLP-1 (7-36) and BRET subsequently measured at the indicated times.
References
1- Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. 2012. Br J Pharmacol. Mar;165(6):1717-36. PMID: 21699508.
2- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. 2000. J Biol Chem. 275(22):17201-10. PMID: 10748214.
3- Wan M, Zhang W, Tian Y, Xu C, Xu T, Liu J, Zhang R. Unraveling a molecular determinant for clathrin-independent internalization of the M2 muscarinic acetylcholine receptor. 2015. Sci Rep. Jun 22;5:11408. PMID: 26094760.
4- Chen Z, Gaudreau R, Le Gouill C, Rola-Pleszczynski M, Stanková J. Agonist-induced internalization of leukotriene B(4) receptor 1 requires G-protein-coupled receptor kinase 2 but not arrestins. 2004. Mol Pharmacol. Sep;66(3):377-86. PMID: 15322228.
5- Namkung Y, Le Gouill C, Lukashova V, Kobayashi H, Hogue M, Khoury E, Song M, Bouvier M, Laporte SA. Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. 2016. Nat Commun. 7:12178. PMID: 27397672.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of βarrestin-dependent seven transmembrane receptor signaling. 2011. Trends Biochem Sci. 36(9):457-69. PMID: 21764321.